Paradromics Successfully Tests Brain Implant in Human, Marking a Milestone in Neural Tech
Paradromics, a Texas-based brain-computer interface (BCI) startup, has achieved a major breakthrough in neurotechnology. The company announced that it successfully implanted its Connexus brain device into a human patient for the first time — and removed it safely just 10 minutes later.
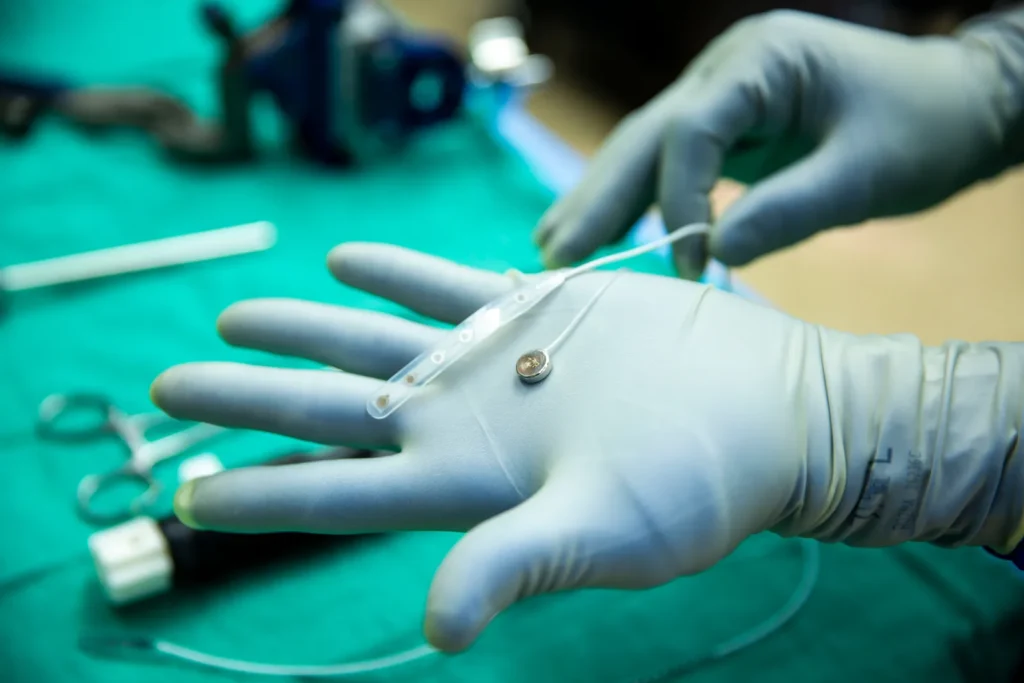
The test, conducted on May 14 at the University of Michigan, marks Paradromics’ transition from animal trials to human testing. The opportunity arose during an epilepsy-related brain surgery, where a consenting patient allowed the device to be temporarily implanted in the temporal lobe, an area responsible for memory and auditory processing. Surgeons used a custom, EpiPen-like tool to place the device, and researchers confirmed it was able to record electrical activity from the brain.
“When a patient is already undergoing a major neurosurgical procedure and part of the brain is being removed, the added risk of testing a brain implant is minimal,” said Matt Angle, CEO of Paradromics.
The Connexus implant is smaller than a dime and features 420 ultra-thin electrodes on needle-like protrusions designed to penetrate brain tissue and record activity from individual neurons. This high-resolution data is essential for decoding complex neural signals such as those involved in speech.
Paradromics is targeting a transformative use case: restoring communication for individuals with ALS, spinal cord injuries, or stroke-related paralysis. The implant is designed to translate brain signals into synthesized speech, text, or cursor movements, potentially restoring autonomy to people who can no longer speak or type.
While Paradromics and Elon Musk’s Neuralink both use direct brain implants, other companies are taking less invasive approaches. For example, Precision Neuroscience is testing surface-level implants, and Synchron uses a device inserted through blood vessels. These systems capture signals from groups of neurons, whereas Connexus records from individual ones — a distinction that offers greater precision.
“Being closer to individual neurons gives us higher-quality signals,” Angle explained. “That level of detail is key for decoding something as complex as attempted speech.”
BCIs like Connexus don’t read thoughts. Instead, they detect and interpret the motor signals generated when a person attempts to move or speak — even if their body can no longer perform those actions. For example, someone who is paralyzed but tries to form words with their mouth still produces identifiable brain signals. These can be translated into real-time communication.
In 2023, researchers at Stanford and UC San Francisco demonstrated BCIs that could decode intended speech at rates of 62 to 78 words per minute in paralyzed patients — about half the speed of normal conversation. Paradromics aims to match or surpass those results.
The company plans to launch a long-term clinical trial by late 2025, focusing on individuals with severe paralysis. These participants would receive permanent Connexus implants to evaluate safety, performance, and usability over time.
“This initial test was about validating the process — getting the system into the operating room, ensuring it works, and confirming it can be removed safely,” said Jennifer Collinger, a BCI researcher at the University of Pittsburgh. “It’s a dress rehearsal for what’s next.”
Paradromics is part of a new wave of BCI innovation building on the legacy of the Utah array, a widely used but aging brain implant. While the Utah array helped paralyzed individuals control robotic limbs and generate speech, it required bulky external hardware and posed long-term durability issues.
By contrast, Connexus is fully implantable, minimally obtrusive, and built for higher performance. Future plans include exploring the feasibility of using multiple Connexus devices in a single brain to boost data collection and system functionality — but for now, the focus is proving that one device can work safely over time.
“Bringing any medical device to market is hard,” said Justin Sanchez, a neurotechnology expert at Battelle. “But when it comes to fully implantable brain tech, proving safety and signal fidelity in a real human brain is the first and most critical step.”